Photocatalytic U(VI) Reduction Assists to Extract Uranium from Seawater
Updatetime:2021-04-22From:
【Enlarge】【Reduce】
As a key element, uranium is widely used as the nuclear fuel in most nuclear reactors. However, it is reported that conventional uranium reserves may be depleted within one hundred years. For this reason, the mining and recovery of uranium has been identified as a critical concern.
Since the total uranium amount in oceans is nearly a thousand of times higher than the land-based reserves, extracting uranium from seawater has attracted numerous attentions over the past decades.
The photocatalysis method showed significantly high extraction capacity, selectivity, and efficiency for the separation of U(VI), which identified as an efficient alternative for seawater uranium extraction in recent years.
However, seawater contains ~2.75 mM carbonates, which would severely inhibit the photocatalytic reduction of U(VI), restricting the practical application of this method.
In this study, scientists from Northwest Institute of Eco-Environment and Resources (NIEER) of Chinese Academy of Sciences (CAS) were devoted to realizing the photo-reduction of U(VI) in carbonates-containing system by developing a highly efficient photocatalyst.
They prepared carboxylated carbon nitride (CCN) catalysts with different amounts of carboxyl groups (CCN-5 and CCN-24) by oxidating bulk carbon nitride (BCN).
Research results indicated that, compared to BCN, the adsorption ability of CCN-24 for U(VI) increased by 30% and 9% in the presence of 2.0 mM and 10.0 mM NaHCO3, respectively.
Moreover, the modification of electron-withdrawing carboxyl groups reduced the conduction band position (Fig. 1A) and promoted the separation efficiency of electrons and holes, facilitating the photocatalytic performance for the reduction of U(VI).
Besides, under the illumination of visible-light, CCN exhibited high reactivity for the photocatalytic U(VI) reduction in carbonates-containing system. In the presence of 10.0 mM HCO3-, CCN-24 showed a U(VI) reduction rate of 0.0867 min-1, being ~33 times of that for BCN (0.0026 min-1) (Fig. 1B).
Electron spin resonance (ESR) and radical trapping investigation confirmed that ?O2- was the main active reduction species for U(VI), and more ?O2- and fewer oxidizing ?OH radicals were generated on CCN than BCN (Fig. 1C and D).
X-ray photoelectron spectroscopy (XPS) and X-ray diffraction (XRD) suggested that U(VI) was finally reduced to U(IV) and UO2+x was finally formed on the photocatalyst surface.
This work opens up the possibility of extracting uranium from carbonates-containing solutions via photocatalysis method, expending the application of this method in natural systems.
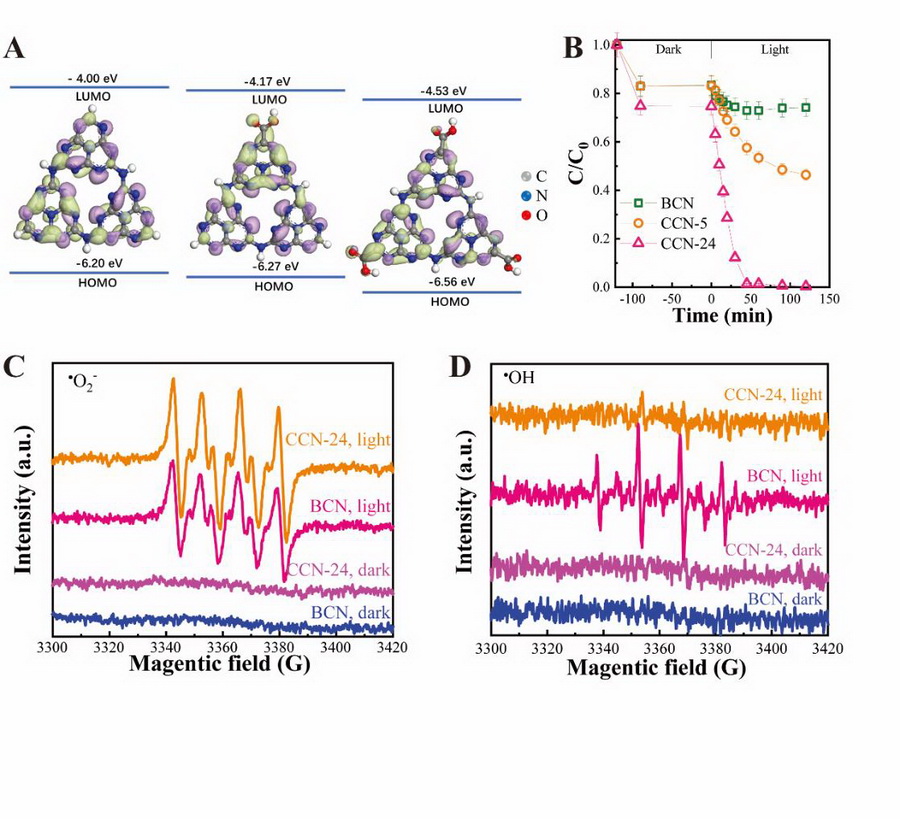
Fig. 1 Molecular orbital energies and structures of raw and carboxylated g-C3N4 (A), The photocatalytic reduction of U(VI) in the presence of 10 mM HCO3- (B), ESR spectra of radical adducts trapped by DMPO (?OH and ?O2?) in BCN and CCN-24 dispersion in the dark and under the visible light irradiation (C, D) (Image by Prof. FAN Qiaohui)
Contact:
Qiaohui Fan
E-mail: fanqh@lzb.ac.cn
Northwest Institute of Eco-Environment and Resources, Chinese Academy of Sciences, Lanzhou, China.
Appendix




