International Collaboration Unmasks 3D Structure of A Key Drug Target for Diabetes
Updatetime:2013-08-13From:
【Enlarge】【Reduce】
Glucagon is a hormone secreted by α cells in the pancreatic islets. It was isolated, crystallized and structurally determined in 1953. Human glucagon is a single chain polypeptide consisting of 29 amino acids (molecular weight = 3485). It starts with a histidine at the N-terminal and ends with a threonine at the C-terminal. Glucagon binds specifically to its cognate receptor (a class B GPCR) located mainly on the surfaces of liver and kidney cells, thereby activating the downstream signal transduction pathway and exerting physiological actions.
Unlike insulin, glucagon increases blood glucose levels via promotion of glycogenolysis and gluconeogenesis. Of various contributors that affect glucagon secretion, blood sugar is the key factor: an increased release is seen when blood glucose is elevated, or vice versa if the latter drops. Insulin can indirectly stimulate glucagon secretion by reducing circulating glucose concentrations. They are thus a pair of hormones with opposing effects and form a negative feedback loop to regulate blood glucose. Therefore, glucagon receptor is well-recognized as a potential drug target for type 2 diabetes.
GPCRs are associated with a variety of diseases and hence, have become targets for many modern medications. There are six subclasses of GPCRs ranging from A through F. However, the three dimensional structures of the GPCRs solved so far all belong to class A. Little is known about the atomic make-up of class B GPCRs.
An international team consisting of scientists from China, USA, the Netherlands and Denmark formed a close collaboration working around the clock for over two years in this effort. A small molecule ligand was used to stabilize the receptor protein and to help crystal growth. The resulting structure the 7-transmembrane domain of glucagon receptor was subsequently determined to a resolution of 3.4 Angstroms. Further studies were carried out with the crystal structure and 128 mutated receptors to analyze changes in binding properties.
It turned out that glucagon receptor has two important features that differ from those seen in class A GPCRs. One is an unusually elongated, stalk-like segment that connects the transmembrane region to the outermost, knob-like domain of the receptor. The other is an unusually large “pocket” within the transmembrane region where the N-terminal part of the glucagon peptide is expected to dock. Based on this new information and previously published work, a detailed model for how the full-length glucagon receptor operates was proposed.
The internationally renowned journal Nature online published the three dimensional structure of the 7-transmembrane domain of human glucagon receptor on July 18, 2013 (Beijing time). The work was completed by scientists from the Scripps Research Institute (USA), the National Center for Drug Screening/Shanghai Institute of Materia Medica, Chinese Academy of Sciences, among others. This signifies a new era in the structural study of class B G-protein coupled receptors (GPCRs) where previous efforts were largely in vain.
This project, led by two national "Thousand Talents Program" experts (Professor Raymond Stevens and Professor WANG Mingwei ), received financial support from National Institutes of Health (USA), China's National Mega Program on Drug Discovery, etc. The same issue of Nature also published the structure of another class B GPCR - human corticotropin-releasing factor receptor 1 unveiled by a British research group. "These reports represent a tremendous breakthrough in GPCR biology", commented by an acompanying News & Views of the journal.
Nature news&views: http://www.nature.com/nature/journal/vaop/ncurrent/full/nature12413.html
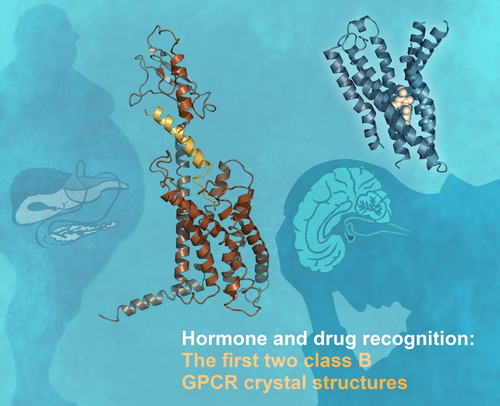
The first two class B G-protein coupled receptor (GPCR) crystal structures (Images by Nature)
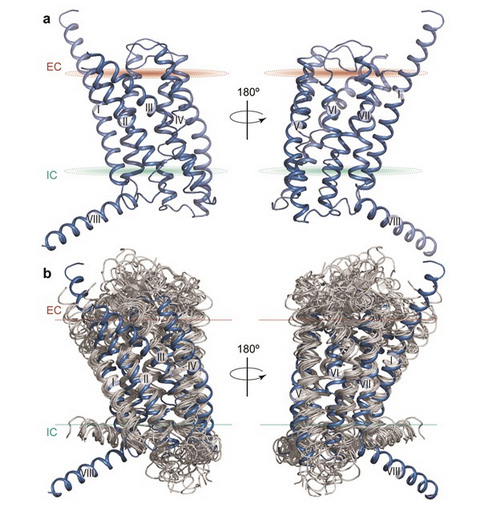
Structure of the 7-transmembrane domain of human glucagon receptor and comparison to class A GPCR structures (Images by Nature)
Appendix




